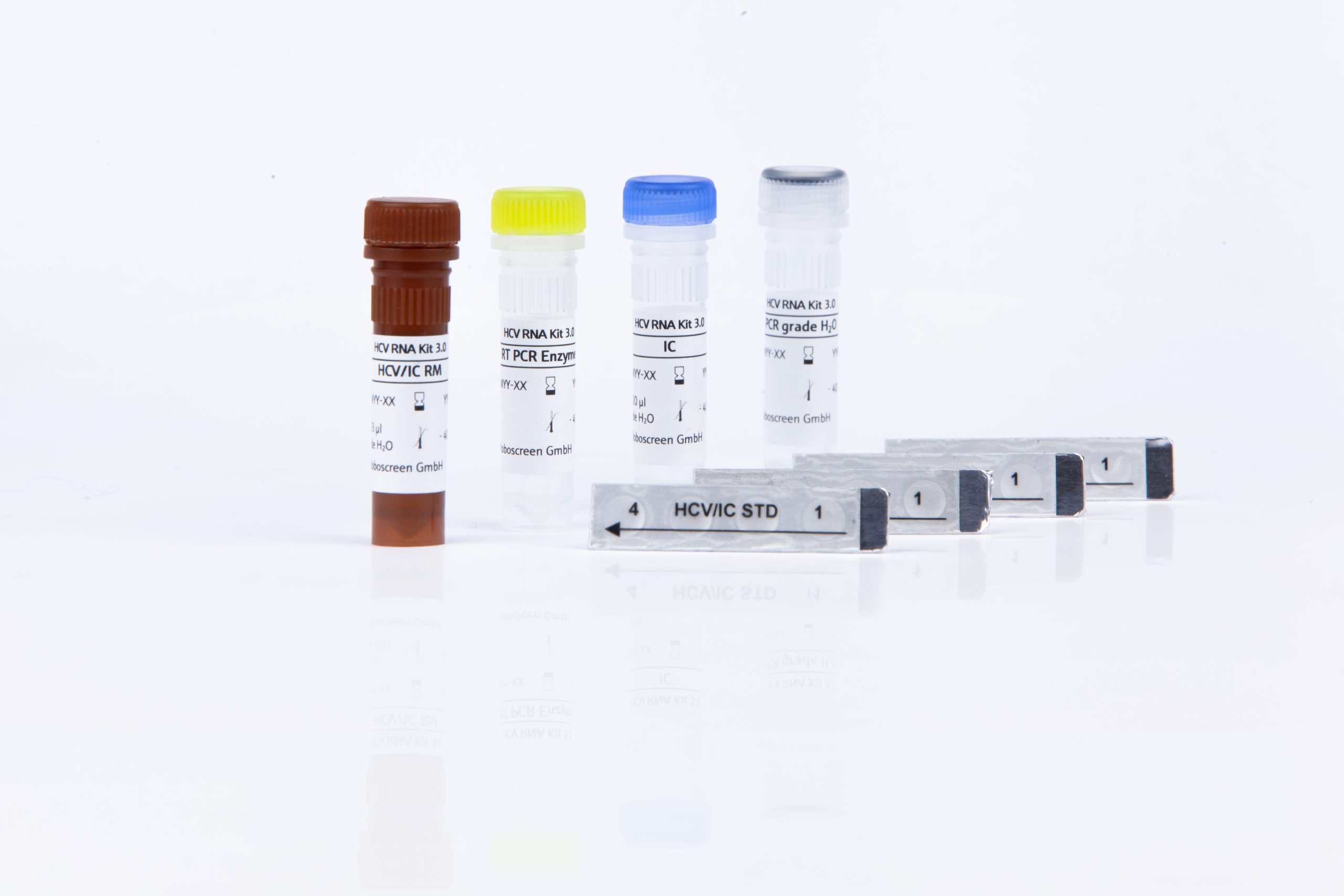
The RoboGene HCV RNA Quantification Kit 3.0 is intended for Real-Time PCR quantification of Hepatitis C Virus (HCV) RNA in human EDTA- or citrate plasma and serum samples. For specimen purification the manual method (INSTANT Virus RNA/DNA Kit) as well as the automated method (INSTANT Virus RNA/DNA Kit – FX) is validated.
The assay is purposed for the clinical management of patients with chronic HCV in conjunction with clinical presentation and other laboratory markers for HCV infection.
This test is intended to assess viral response to antiviral treatment as measured by changes in plasma and serum HCV RNA levels. Furthermore, in a course of antiviral therapy the probability of a sustained viral response can be judged.
The RoboGene HCV RNA Quantification Kit 3.0 is not intended for use as a screening test for the detection of HCV RNA in blood or blood products or as a diagnostic test to confirm the presence of HCV infection.